
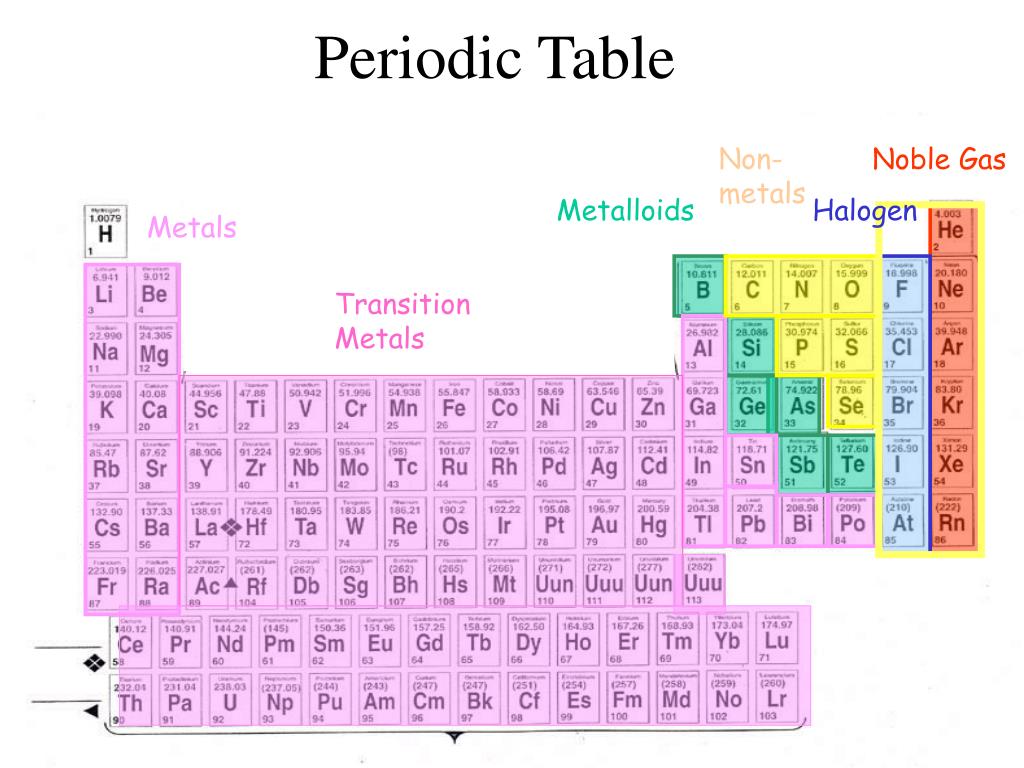
Remember, gaining an electron is favourable for halogens because it enables them to form an anion with the same electron configuration as a stable Group 18 (Noble Gas) element. This is why we say that the properties of group 17 elements become more metallic in character as you go down the group from top to bottom, even though all the elements in group 17 are non-metals.įirstly, all the halogen atoms are very electronegative, they are all very capable of pulling an electron towards themselves. Pale yellow to greenish-yellow to reddish-brown to gray There is also a gradation in the colour of the elements going down group 17 from top to bottom: We can infer that there is a gradation in the intermolecular forces acting between the molecules such that the strongest forces of attraction act between iodine molecules and the weakest forces of attraction act between fluorine molecules. The melting point of a substance reflects the amount of energy required to weaken the forces of attraction between molecules ( intermolecular forces), the higher the melting point the stronger the forces of attraction between the molecules. Going down Group 17 from top to bottom the elements change from gaseous state to liquid to solid. We can see a trend in the states of matter. Summary of trends in the properties of Group 17 elements is shown below: Metallic character of the group 17 elements increases down the group from top to bottom.Elements become darker in colour going down group 17 from top to bottom.Melting point and boiling point increase down Group 17 from top to bottom.First ionization energy decreases down group 17 from top to bottom.Chemical reactivity of group 17 elements decreases down group 17 from top to bottom.Electronegativity decreases down group 17 from top to bottom.Atomic radius increases down Group 17 from top to bottom.All Group 17 (group VIIA or halogen) elements have 7 valence electrons (7 electrons in the valence shell or highest energy level).(ii) covalent (eg, non-metal + halogen → non-metal halide) (i) ionic (eg, metal + halogen → metal halide)

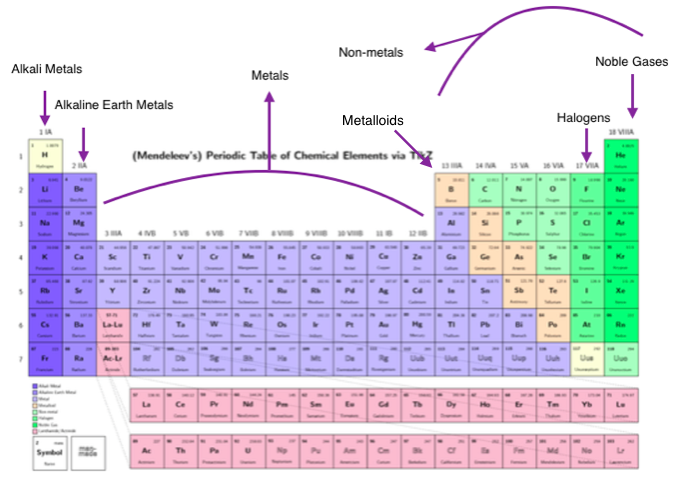
‣ Are noble gases main group elements?Īll noble gases are main group elements.(a) halogens (2) (name still in common use) Sodium is a main group element located in period 3 of the periodic table. ‣ Is copper a main group element?Ĭopper is a transition metal, not a main group element. The cerium (Ce) is a lanthanide, not a main group element. ‣ Is calcium a main group element?Ĭalcium is a main group element located in period 4 of the periodic table. Sulfur is a main group element located in period 3 of the periodic table. The caesium (Cs) is a main group element located in period 6 of the periodic table. The main group elements with an odd atomic number include the alkali metal family, boron family, nitrogen family, and halogen family. ‣ Which elements is a main group element with an odd atomic number? Oxygen is a main group element located in period 2 of the periodic table. Thallium is a main group element located in period 6 of the periodic table. The elements of s-block and p-block together make a boron group element. The lead (Pb) is a main group element located in period 6 of the periodic table. ‣ Is aluminium a main group element?Īluminium is a main group element located in period 3 of the periodic table. Gold is a transition metal, not a main group element. – main group element FAQs on Main Group Element ‣ Is gold a main group element?


 0 kommentar(er)
0 kommentar(er)
